PHARMALINK Resources > Webinars
PharmaLink Webinars
In February of 2022, the efforts of Xavier Health were assumed by the AFDO/RAPS Healthcare Products Collaborative. Because of the important work done before this transition, the Collaborative has chosen to retain documents that have Xavier branding and continue to provide them to the communities through this website. If you have questions, please contact us at info@healthcareproducts.org.
Predictive “Quality” for Patient Safety Webinar
A free webinar presented by Mgmt-Crtl and Compliance Group

Larry Mager
Principal and Founder, Mgmt-Ctrl

Marla Phillips
Director, Xavier Health, Xavier University
Webinar Description
Predictive Quality Management is an emerging category of industrial Artificial Intelligence solutions that provide manufacturers with a means to significantly reduce process-driven losses in quality and waste, by quickly identifying the root cause and, with a high degree of confidence, preventing those losses before they next occur.
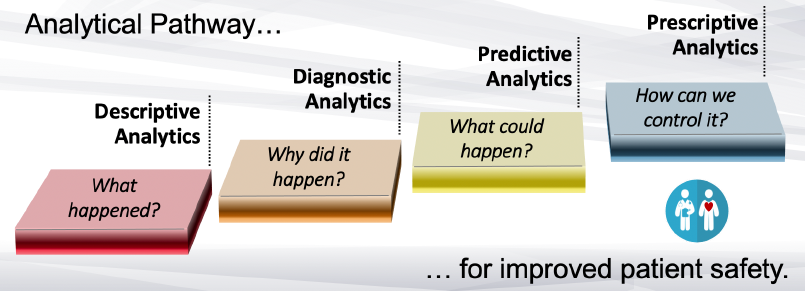
Predictive “quality” for patient safety is focused on the management and reduction of patient harm that is associated with the use of a medical device.
Learn more about the predictive “quality” approach for the post-market management of patient harm, including the utilization of an analytical pathway to predictive ‘quality’ for improved patient safety.
Phase Appropriate GMPs for GCTs Webinar
A free webinar presented by Compliance Insight

Troy Fugate
Vice President, Operations, Compliance Insight

Melissa Schneider
Manager, Regulatory Compliance, Compliance Insight
Webinar Description
This free webinar will outline Good Manufacturing Practices (GMPs) for Gene and Cell Therapies (GCTs) that will enable you to:
- Get started in development – understand when too much is too much, and when too little is too little
- Avoid late phase/commercialization disasters by planning now
- Avoid delays in batch releases through proper risk mitigation planning
- Understand when you should validate methods or processes

